Chemistry student searches for eco-friendly solutions

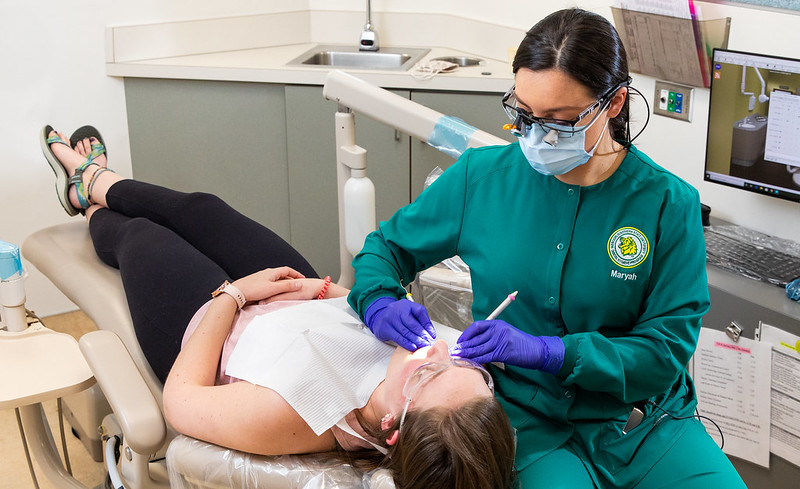
Senior biochemistry major Lauren Sanders, with the help of her adviser Dr. Elliot Ellis, measures out the chemicals needed to test compounds featured in her senior thesis project over ionic liquids in Reynolds Hall on Sept. 8.
An interest in molten salt doesn’t seem like a very likely start to a career, but for Lauren Sanders, it may be the gateway leading her to a career in pharmaceutical research.
The senior biochemistry major is beginning work on her honors senior thesis project involving room temperature ionic liquids, better known as molten salts.
“An ionic liquid is a salt with ions that aren’t strongly coordinated,” Sanders said. “They are very customizable … so there are endless possibilities of what you can do with them.”
One use for such ionic liquids in is anti-fungal medication used for problems such as athlete’s foot and ringworm. Sanders is hoping to use her research to expand the use of these ionic liquids as anti-microbial agents in the medical field.
Watching her in the lab, a visitor would simply see Sanders measuring different chemicals into beakers. But what she’s actually doing is far more complex. She is looking at the effects that different chemicals have on these molten salts. In the process, she weighs the substance and measures the makeup of the compound.
“The goal isn’t as much to find one specific one, but we will test them and look at toxicity and characteristics of them and see how they can work that way,” Sanders said.
Sanders is still early in the process. Because there are so many possibilities, Sanders needed to narrow down her options, and is focusing only on certain carbon chains. With the help of her adviser, Dr. Elliot Ennis, Sanders will test 18 different chemical combinations to see what they can do.
“This could potentially make them more eco-friendly, but it depends on how we change the carbons,” explained Ennis. “ We’re just taking the same idea and hitting at every angle we can and trying to find out what makes it tick.”
Your donation will support the student journalists of Missouri Southern State University. Your contribution will allow us to purchase equipment and cover our annual website hosting costs.